Surely every welder often wonders how to get rid of rust on metal once and for all. Working with welding is always associated with such a problem as corrosion of metal parts.
This problem is especially relevant when repairing cars, when you have to weld the body and perform other related work. The next step is painting. This is a critical stage and requires thorough preparation of the metal.
Today you can find many products on sale that will help remove rust and protect against it. First, the rust is removed until the metal shines, and then the surface is coated with a special converter. Such a converter can not only get rid of rust, but also protect the problem area from corrosion for some time.
Rust converter composition. Can I do it myself?
This article is a continuation of the material about painting rear brake drums . I already said that before the painting process you need to treat them with a rust converter. I just didn’t say what it is and how it works, because the article is about something else. It doesn’t matter, in this material I will put everything on the shelves, and the converter is used not only before painting work. It can be used as a protective agent for areas already eaten away by rust. The composition is really interesting, and you can repeat it yourself...
As it becomes clear, he fights rust, removing or, if you like, transforming it. When the product is applied to a “damaged” surface, a reaction occurs. The rusty place, so to speak, is “cleaned”, that is, there are no rusty spots on it, but this does not happen until the metal shines. And it is covered with a protective film, which should preserve the surface for a year or two.
A really useful thing, for example, a wing or drum has “bloomed”, that is, pockets of corrosion have appeared, you treat it, and the surface is covered with a light or dark film, which in turn is protection.
Causes of rust
Most often, rust appears after winter.
- Paint chips. During operation, chips will always appear on the paint of the car, from small stones and sand. If the chip is deep enough and metal is exposed, then rust will appear on it within a month. If this place is not treated, then in a year, if the quality of the metal is poor, such a chip can turn into a hole.
- Dents and scratches. If the car body has been deformed, this can also lead to rust. The paint cracks at the bends, and moisture gets into the cracks, causing corrosion. If such cracks and scratches are not treated in a timely manner, rust will appear here over time.
- Excess moisture inside the car. Moisture should not accumulate inside the car, otherwise it will lead to corrosion on untreated parts. At the same time, the fight against such rust on a car is very complicated, since all the parts are covered with casing from the inside, and you simply cannot see how they rust.
- Lack of anti-corrosion treatment in winter. In winter, to remove snow, road services use chemicals that are very harmful to the car body. When they come into contact with untreated metal, they lead to accelerated corrosion, and they also destroy the paintwork.
How does it work
The process is simple. You buy a converter; it usually comes in spray cans. Next, it is sprayed onto the site of the outbreak, then it is advisable for us to leave this place for about half an hour - an hour.
The product does not remove rust at all, if I may put it roughly, it freezes it and prevents it from spreading further. An iron phosphate film forms on the affected surface, which is a protective element. The work period is approximately one to two years, then it is advisable to repeat the procedure.
I would like to note from personal experience that my uncle is a minibus taxi driver and works on his minibus almost every season before winter. And you know, it really rots less when compared with untreated neighboring cars along the route.
But we are wondering whether it is possible to produce it with our own hands, what is the approximate composition. I’ll say right away that there are two compositions, the one that is used in production, and the one that is made at home.
Composition in production
The rust converter in production is a rather complex composition; of course, no one will show you a specific formula, but it’s a secret. But you can roughly find out.
So, there are several main components:
- Phosphoric or orthophosphoric acid
- Distilled water
- Zinc (liquid)
- Sometimes tannin
- Ascorbic acids
- Biocides
- Pigments
- Inhibitors
- All kinds of surfactants
It is worth noting that sometimes all these components are concentrated in one “bottle”, but sometimes not. Therefore, compositions are usually distinguished into subgroups.
- Simple converters, they are designed to remove rust from the surface and create slightly soluble salts.
- Converters - stabilizers. They modify rust into stable compounds.
- Primers. As a result of exposure to such a substance, a layer of durable protective primer is formed on the surface, onto which paint can be applied. That is, it is an excellent anti-corrosion film.
I would also like to note that converters can be supplied in various forms. That is, they can be in the form of aerosols, in the form of a gel, or simply liquid in bottles. In bottles, these are the simplest and cheapest options, but they cost the most; gel and aerosol components are more expensive, but their consumption is much lower.
Chemicals for rust control
Removing rust from a car body with your own hands
The most popular industrial rust removers are chemical solvents made using phosphoric and oxalic acid. Each of these products must be used according to individual instructions, but the general scheme is almost the same: first, the flaking rust is cleaned off, then a layer of the product is applied to the affected area and left for the time stated in the instructions. After this, the surface is finally cleaned of rust.
Chemical solvents confidently cope with corrosion
A new product that is becoming increasingly popular is the rust converter. This product, produced in the form of a spray or suspension, can preserve rust and stop the development of the corrosion process. Before applying a rust converter to the surface, you need to clean the metal and then apply a product that restores the structure of the iron. The negative point is that the metal surface after this treatment acquires a purple tint and therefore needs to be painted.
How to make it yourself
The easiest way is to find phosphoric or orthophosphoric acid and mix it in the required proportion with water, the proportions are approximately 60 to 70% acid, the rest is water. This will already be enough to remove a layer of rust from the surface.
However, it is not always easy to find this component, and you don’t always want to spend money on a converter, because, for example, you just need to treat the brake drum to protect the surface before painting.
Then you can make it yourself from available components, and almost every home has them, we will need:
- 1 liter of distilled water.
- Citric (or oxalic) acid. We take about the same amount as water, that is, a liter, maybe even one and a half.
- 15 grams of regular soda.
First, we make a base, that is, we add acid to the water and after that we need to add soda. The reaction should take about 30 - 40 minutes, after it is over, we need to put on a rubber glove, take a rag and soak it in this solution. Next, we simply apply this “rag” to the affected area. Warning, it is advisable to clean it.
You should not expect too strong a reaction; after all, these acids are household ones and do not have high activity, but as an auxiliary agent. For example, if you cleaned a brake drum, you need to treat the surface, it will definitely do. It also cannot fight deep rust.
If you need a product specifically to combat deep lesions, you need to buy a specialized composition, because there is a large number of active substances that are selected according to a strict, secret formula.
Now let’s watch a short video instruction on the use of phosphoric acid.
This is how the article turned out, I think it was useful. Read our AUTOBLOG.
( 12 votes, average: 3.67 out of 5)
Similar news
Is it possible to mix brake fluids? Let's say you produce different ones.
How to unscrew a brake pipe. If it has soured and the edges are torn off.
Anti-squeak plates for brake pads. Why are i needed?
Why does rust occur?
When iron or steel is not protected, a process of reaction with oxygen occurs and corrosion of the metal occurs. The metal breaks down and forms rust, a red-brown compound that is an obvious sign of electrochemical oxidation of the metal. Other metals also oxidize, but they do it differently. So, for example, corrosion of aluminum spreads much more slowly.
Corrosion deteriorates the properties of the metal, its structure and thickness, leading to a loss of strength. Corrosion can be concentrated in individual points, or it can spread over a large area more or less evenly.
The rate at which metal rusts is quite slow, but it is accelerated by contact with water, especially if the water has a high concentration of salt, which acts as an electrolyte (a substance that helps electrons move). Also, the rate at which rust spreads is affected by the ambient temperature. The higher the temperature, the faster the metal rusts.
Why does metal rust? With the exception of gold, platinum and a few others, metals do not occur in nature in pure form. They are chemically combined with other elements in the ore, such as sulfides and oxides. To extract metals from sulfides and oxides, energy must be expended (in a blast furnace) to obtain pure metal. Since all elements in the universe tend to return to their lower energy, ground state, pure metals also tend to return to their ground state in which they were in nature (in the form of sulfides and oxides). The path that returns a metal to its ground state is its oxidation. Thus, corrosion is a natural process that transforms a metal into its chemically more stable form.
Rust does not spread through contact like a biological infection. Instead, the process of iron oxidation occurs independently, depending on environmental conditions.
Different metal alloys rust at different rates, depending on their composition. Impurities in metal alloys accelerate the corrosion process. Pure iron does not oxidize as aggressively. Unfortunately, pure iron is not a very good material for making car bodies. By adding carbon, the result is steel that already has all the necessary properties for sheet metal forming, such as elasticity and tensile strength.
It would be more honest to call the fight against rust a delay from its occurrence. You can't win it completely. Cars from the factory have weak points that “wait” for favorable conditions for corrosion to occur. A vehicle that appears rust-free may contain rust in hidden cavities or on the back of some panels.
Corrosion can occur on a car body in the following cases:
- Due to the appearance of a deep scratch or chip of the paintwork.
- Rust occurs in areas of the body where dirt and salt accumulate. These are areas around the fenders, at the bottom of the doors, due to blocked drainage holes.
- Spot welded areas are also prone to rust.
- Rust usually starts at the edges of the panels. These parts have a thinner layer of paint that wears off faster.
- Thresholds and other hidden cavities can rust from the inside.
- Rust may appear in places where body repairs were carried out and anti-corrosion treatment was not carried out correctly.
vmg1961 › Blog › Rust converter or how to prepare bare steel for painting.
Let's continue our chemical experiments. As is known, in the production of automobiles, after welding work, the body is phosphated or zinc-phosphated or galvanized, and then primed with cathophoresis primer, which in total provides very good protection against corrosion.
Unfortunately, such technologies for local or major body repairs or restoration are not available to us due to the need to have a very specific production base. I want to: Firstly, remove as much corrosion as possible, especially in capillary and crack-like areas. Secondly, create a high-quality base for applying paintwork.
I immediately rejected technologies using electrochemical and hot processes due to their complexity and volume. Small things can still be done, but the car body or individual parts are more difficult. I tried and tested various commercial products such as rust converters - but they didn’t particularly impress me either.
And then one day I discovered an interesting document from the times of the USSR: UDC 621.315.66:667.673(083.96) STANDARD INSTRUCTIONS FOR PAINTING METAL POWER LINE SUPPORTS USING A RUST CONVERTER. It is available on the Internet (for example, here: base.consultant.ru/cons/c...?req=doc;base=ESU;n=14567), so I will not publish it. And I decided to try this technology. And I tried it. I’ll say right away that I liked this method. 1. Simplicity and availability of reagents. Low price. 2. Simplicity of the processing process and predictability of the result. 3. High quality coating.
The most interesting thing is how I carry out the above instructions. So, step by step:
1. We tear, gnaw and scratch or wash off the old paintwork. Feel free to use chemicals and other nasty things. And even sandblasting, although I consider processing with quartz sand unacceptable in body repair, but more on that later - there will be a separate publication. 2. Wash the part with fairy water. Wipe dry. Pour boiling/hot water over it. Wipe dry. 3. Pour the rust converter (the one prepared according to the recipe) into a bowl-type container. 4. Dip red Scotchbrite into it (using rubber gloves, of course - at least at the beginning) and carefully unwind the part to be processed. In the process, the converter becomes dirty, almost black. In one dip I treat an area of 40x40 cm. 5. Wipe the treated area dry with a napkin. 6. P.p. 4 and 5 throughout the detail.
Rust can be stopped. DIY rust converter
The places where rust occurs on cars and how to combat it are described.
In the automotive world, there are many problems associated with the operation of cars, but perhaps the most unpleasant is rust. In some developed countries there are no rusty or scratched cars. Why? - you ask. It turns out that when owners undergo a technical inspection every year, they first eliminate all visible defects - pockets of rust, scratches, dents that spoil the appearance of the car. The owner of a car who cherishes his car and takes care of it understands that corrosion not only spoils the appearance of the car, but also greatly destroys it. An attentive driver should always have at his disposal a variety of rust control agents and use them well.
If you don’t want to throw out the “extra” 50-150 dollars in order to remove the rust that has “appeared” on the body (hood, fenders, doors, etc.), then it is imperative to buy a metal brush and sandpaper. You will need these things before almost all types of work - priming, painting, anti-corrosion treatment of the body. You need to fully identify all the places that cause you suspicion. Well, then go ahead! The entire corrosion spot and a couple of centimeters around it must be cleaned with a brush and then sandpaper until the area is almost perfectly smooth and the metal shines. You can control the quality of stripping by hand.
At the second stage, which is even more important: the thoroughly cleaned area must be treated with a rust converter, which can even be purchased at hardware stores, you just need to make sure that the product is of proper quality and that you are not being given a fake. At this stage you must be extremely careful. Acid is the main component of all rust converters. Therefore, it is best to first test the purchased drug on a previously prepared piece of metal, and only then use it directly on the car. Before use, carefully read the operating instructions and consistently follow everything that is written there. After treatment with a rust converter, it is necessary to degrease the surface and prime it.
You can also make a rust converter yourself. To do this, take 1.5-2 liters of ordinary water and add a little more citric or oxalic acid to it - you will get a saturated solution. Then add fifteen grams of soda there. At the next stage, soak a cotton rag in it and apply it to the cleaned areas, keeping it there for 10-15 minutes. This homemade solution is good not only as a “repair” substance and is also suitable as a material for the prevention of corrosion “diseases”.
Prevention, of course, is best done with special means that are sold in the store, but their cost is quite high. There are cases when car enthusiasts treated the body of their car in this way once a year. There were no complaints from them, despite the fact that their cars are constantly parked on the street. It is advisable to use such a solution from the first year of operation of a new car, in order to completely destroy all the beginnings of rust, but at the same time not touch the paint and metal. Prevention must be carried out as follows: a rag soaked in the solution is applied one by one to the surface of the entire body and left for 10-15 minutes at each place. Bumpers and rims can also be treated in a similar way. After the specified time has elapsed, you need to remove the rags and rinse the remaining solution thoroughly with water, or even better, with dishwashing liquid, such as Ferry.
An even greater effect can be achieved by using a rust converter, which is made on the basis of an electrolytic solution of zinc salt. The work will be more difficult, but the effect will be even better. In this case, the cleaned surface of the spot must be treated with cotton wool, which is wound around an electrode connected to the battery. Having lowered this electrode with a cotton swab into the solution, you need to rub the area in a circular motion until it begins to turn gray. This is zinc, which settles on the surface of the metal and thereby saves it from corrosion. In general, this process can be called galvanizing. After processing, it is imperative to clean these places.
There comes a time when you can apply the primer. Without this operation, neither paint nor anti-corrosion treatment may have an effect - they will quickly disappear. Therefore, before painting or treating with mastic, you need to prime it well and let the surface dry.
Many places that need a protective layer of paint or mastic are in practice difficult to find with the naked eye. A very thorough inspection is required. Let's pay attention to the body. After any “micro collision” with an obstacle, you need to examine the damage and treat it. Even if nothing is visible on the body, water can easily get to the metal through micro cracks in the paint and do its bad work. It is necessary to treat it with a special anti-corrosion substance - there are many of them. It is best to buy these substances in aerosol containers.
If you accidentally snag and tear off the holding, do not rush to put it back together. Apply at least Movil to the places where the molding is attached; most likely they were damaged or scratched.
The body has its own internal part. The lower part, which is under your feet, can especially suffer. You need to check there often enough. Yes, yes, you need to lift the insulation and sound insulation and treat it underneath. Rubber mats, of course, help in winter, but not completely. If you want high-quality processing, then even bother removing the seats. There you can see very unpleasant moments. Thresholds are one of the most vulnerable areas of the body, and especially of domestically produced cars. It is necessary to periodically “blow” anti-corrosion material into the thresholds, for example, the so-called “cannon fat”.
How to remove rust
However, we all have that favorite pot or pan that's a little rusty at the base or handle, but we don't want to throw it away. If this is your case and you have a number of items that you want to restore, keep these tips in mind. They will help you in the fight against rust.
An abrasive sponge and baking soda will help remove rust.
You will find these abrasive sponges in any supermarket, they are very useful in these cases. To ensure effective rust removal, we use our star product: baking soda.
What you need
- abrasive sponge
- baking soda (amount depends on surface area)
What should be done
First, heat some water and then mix it in a bowl with baking soda. You should have a thick paste. Its quantity will depend on the area of the rusted surface.
Dip a sponge into this paste and begin to gently scrub the pan.
Please note that if you do this too intensely, you may ruin it.
Rub gently and then let sit for 5 minutes.
After this time, rinse the dishes with clean water.
The next step is very important. It ensures that the pot or pan remains in good condition until the next use.
You should dry the dishes well with a paper towel. Once this is done, apply a small amount of olive oil to the surface. This will create a protective layer.
The result will pleasantly surprise you.
Coarse salt and lemon
This method is effective, fast and, above all, very economical. In addition to cookware, rust can be found in other places, such as the kitchen sink. During use, due to hard water, the surface gradually loses its shine. Rust usually accumulates in hard-to-reach areas, such as around the faucet, or in corners where moisture usually remains. To prevent this, use this simple recipe.
What you need
- Coarse salt (amount depends on surface area)
- ½ lemon
What should be done
Take a lemon and cut it in half. Then apply some coarse salt on it. Use lemon as an abrasive sponge.
- After removing food debris from your pans or other utensils, rub them well with lemon. When you see the salt has darkened, add a little more.
- Gradually you will see that the rust is going away. Let the dishes sit for about 5 minutes and rinse off the salt with water.
- Finally, complete the cleaning process in the same way as in the previous case: dry the surface with a paper towel and wipe with olive oil.
- In case of washing, simply wipe it with a paper towel. It will immediately shine like new.
We restore cutlery that is rusty from time to time
It is possible that your family has “heirloom” kitchen utensils, but they have become rusty and you want to restore them.
Is it possible to remove rust from them? Certainly. Next we will tell you how.
What you need
- Aluminium foil
- Juice of one lemon
- White vinegar
- What should be done
The first thing we will do is remove as much rust as possible. To do this, cut the foil into 3cm wide strips. Roll them into small “balls” and dip in lemon juice.
Then start rubbing dishes or other surfaces with them.
After we have removed most of the rust, we move on to the second stage. Place the object in a container of white vinegar until it is completely covered.
Leave it there overnight. The next day, use the recipe with baking soda and an abrasive sponge to finally remove any remaining rust.
If there is too much rust and our advice has not helped, then there is no choice but to use strong chemical and abrasive products, such as hydrochloric acid.
In conclusion, remember that the best way to combat rust is to prevent it from occurring. Published by econet.ru
PS And remember, just by changing your consumption, we are changing the world together! econet
Methods for removing rust from a car
Today there is a wide range of such chemicals on sale. However, the issue of making anti-rust products on their own is of interest to many.
Having decided to use a homemade converter, you must follow the recipe and technology for its use. At the same time, it is worth remembering all the pros and cons of such a product, as well as precautions when using it.
We'll tell you how to make a rust converter with your own hands at home in this article.
Can it be done at home?
The purpose of any rust converter is to convert iron oxides into inert compounds that form a hydrophobic film on the metal surface.
In order to achieve this goal, manufacturers select a specific chemical composition. Each company has its own secret nuances, which it tries not to disclose , so as not to provoke the creation of fakes.
However, the basis of any recipe contains a standard ingredient - phosphoric acid. It reacts with oxides and participates in the formation of a protective phosphate film.
In addition, the converter may include:
- tannins (convert iron oxides into neutral tannate complexes);
- zinc compounds (after reaction with phosphoric acid forms a protective layer);
- corrosion inhibitors (form an additional film, slowing down the corrosion process).
At home, it is easy to find analogues of the main active ingredients and prepare a simple but effective converter. It is prepared from a smaller number of components by conventional mixing in a container.
Chemical cleaning
The arsenal of chemical cleaning methods and compositions is most diverse. Corrosion is removed chemically - by treating the oxides with acid solutions.
- The most effective remedy is hydrochloric acid, especially at an appropriate concentration - at least 15% (at a lower concentration, the dissolution does not stop, but slows down noticeably).
- The use of compositions based on sulfuric acid is not recommended, since as a result, a layer of iron hydrides is formed on the cleaned surface, which increases the fragility of the product. This layer does not need to be cleaned: after a day, this chemical compound will disintegrate on its own under the influence of moisture in the air. However, sometimes this is not acceptable.
- At home, you can use vinegar, lemon, or even Coca-Cola to remove rust from small surfaces. The principle of operation is the same - we immerse the object in acid and leave it for a while, then clean it.
The etched surface must be neutralized from acid residues - washed under a warm soapy solution, and then thoroughly cleaned or dried in calm air.
Pickling is an environmentally harmful process: care must be taken to ensure that the remnants of the used product do not get on food products, protect your hands from the corrosive effects of reagents, etc. Therefore, the liquid that can be used to effectively remove rust must be non-toxic. The following methods can be used as an alternative to acid etching:
- Electrolytic etching in ferric chloride solution. This composition is non-toxic and available for purchase. Cleaning is carried out by immersing the item with rust in a solution of ferric chloride, while connecting one of the electrodes to the bath with the solution, and the second to the item being cleaned. The voltage during electrolytic etching is low - no more than 12 V, so the technology is completely safe. In the absence of ferric chloride, you can use any household alkali, in particular caustic soda. Depending on the required cleaning intensity, the process can be continued for 3-15 minutes.
- Applying a special cleaner to the metal surface, which includes borax, lime, and rust converters. The mechanism of action of such a substance assumes that its components penetrate into the sublayer part of the rust, soften it and then transform it into flakes, which can be easily removed with a stream of warm water.
The choice of the optimal method for cleaning metal from rust depends on the size of the item, the availability of tools and equipment. A well-cleaned surface must be further primed, and it is ready for painting.
Rust removal
Replacing the VAZ 2110 thermostat yourself
You can make the product suitable for use by removing the resulting rust at home. Rusty deposits can be successfully removed from household items, such as knives, using the following home remedies or special preparations made in industrial conditions.
Acidic solutions
It is most convenient to use anti-rust vinegar at home, since almost everyone has this product. To do this, you need to immerse the rusty object in vinegar for some time, for example, overnight. Then remove and wipe the rusted area with a hard cloth or crumpled foil. You can use table vinegar, but apple cider vinegar is more effective.
Lemon juice or lime juice is also used to clean metal. It is necessary to pour salt onto the problematic part of the knife, moisten it with juice, leave it for a while, then wipe off the deposit with aluminum foil.
Phosphoric acid converts rust into iron phosphate or a black coating that is easily removed. This chemical is not always available in its pure form in everyday life. The acid is found in small amounts in molasses and the fizzy drink cola. You can leave a rusty object in a vessel with cola for a while and see the result.
Hydrochloric acid is good at corroding rust. This aggressive liquid is included in some household cleaners, which can be used to rub a damaged metal object and leave it for a while.
Oxalic acid is found in raw potatoes. You can leave the knife stuck into the potato for several hours. Or you can cut the root vegetable, sprinkle the cut surface with soda and rub the rusty area. After this, complete the process using a metal sponge.
Cleaning pastes
Baking soda paste has a good effect. To do this, you need to mix soda with water to obtain the consistency of toothpaste, then apply it to a rusty knife and rub it with a metal sponge. You can repeat the process to achieve the desired result.
Another type of homemade paste is hydrogen peroxide paste with cream of tartar, which has the same consistency as soda. To do this, the composition must contain more cream of tartar. Instead of hydrogen peroxide, you can use water, since the active element that corrodes rust is cream of tartar.
Electrolysis method
This method is not as difficult as it seems. The sequence of actions when using electrolysis to remove rust is as follows:
- Fill a plastic container with water so that the item can be completely submerged.
- Add 1 tablespoon of baking soda to 4 liters of water and stir well.
- For the sacrificial anode, select a large metal object so that half of it is above the water. During the electrolysis process, rust will settle on the anode. Aluminum or stainless steel should not be used.
- Connect the negative pole of the battery charger to the rusted object with a clamp. To do this, select a rust-free area.
- Place the completely rusty object in the water so that it does not touch the anode.
- Connect the positive terminal of the charger to the sacrificial anode.
- Turn on the charger and leave for up to 20 hours.
- Disconnect the charger and remove the wires. For final removal of plaque residues, it is necessary to use an abrasive sponge.
Finished chemicals
Rust removers can be purchased at a hardware store or auto supply store. The following brands can be offered to choose from: “Acid Magic”, “Evapo-rust”, “WD-40” (or the so-called “Vedeshka”), and others. They are convenient and effective to use. Some chemicals come in a spray can, while others come in a solution that you apply with a brush. Another way is complete immersion in a liquid product. Sometimes severely rusted items require refinishing.
Mechanical abrasives
To remove corrosion from large metal surfaces, you can purchase a hand grinder. This power tool is sold in hardware stores. Some large stores provide such tools for rent. Grinding machines are equipped with removable discs that can be replaced when worn out.
How to remove rust from plumbing fixtures
Collection of tips for car enthusiastsDo-it-yourself car repairDo-it-yourself car maintenance
It is easier to prevent the appearance of rust on household plumbing than to deal with it later, so you need to immediately remove rusty streaks and stains that have formed on the surface of the bathtub, toilet or sink. At the same time, do not forget about regular cleaning of plumbing elements with detergents, and avoid stagnation of water and prolonged contact with metal objects.
If a rusty stain or leak does form, a warm vinegar solution will help. To prepare it, heat 1 glass of vinegar in an enamel bowl, then add 0.5 teaspoon of salt and stir. The resulting solution should be treated with the surface of the plumbing fixtures and left for 3-5 hours, depending on the degree of complexity of the rust. After removing the stain, the mixture should be washed off and the treated area dried with a dry cloth.
It is best to prevent the formation of rust on plumbing fixtures
A good solution is also a solution of ammonia and hydrogen peroxide, which is prepared in a ratio of 10:1. You need to treat the stain with the product and leave it for a couple of hours, then rinse and dry the treated area. If the stain has recently formed, you can use less aggressive means - for example, grease. The stain is treated with butter or lard and left for 5-6 days, and then wiped with ammonia. This treatment will protect the surface from rust for a long time.
Household plumbing fixtures are also cleaned with a liquid soda solution. To prepare it, 2.5 tbsp. Soda is mixed with a liter of hot water, the solution is applied to the rust and carefully cleaned. If necessary, the procedure can be repeated several times. It is worth noting that abrasive substances, which include soda, salt and ready-made chemicals with small particles, are used exclusively for plumbing fixtures with an enamel surface. They need to be applied without unnecessary pressure, using a soft sponge.
If you need to clean acrylic plumbing fixtures, you should never use abrasive products - this will only ruin the surface. In this case, you can use mild substances such as lemon juice or vinegar solution. Thus, there are many ways to get rid of rust stains from almost any surface, the main thing is to strictly follow the manufacturer’s instructions and adhere to the recipe when making products at home.
Pros and cons of a homemade product
The anti-rust product, made by yourself, has a number of advantages. However, it cannot completely replace professional converters. Due to the existing disadvantages, many users prefer ready-made store-bought chemicals. For an objective assessment, it is necessary to consider both the pros and cons.
Homemade composition is valued for the fact that it:
It is not toxic to humans, as it consists of harmless substances.
- Does not require very complex and time-consuming manufacturing technology.
- Has simple and clear instructions for use.
- Helps achieve rust removal and coating protection.
- It is much cheaper than branded analogues.
How to make, what is included?
Any converter must contain two main substances that react and form a protective film on the metal surface.
Home remedies that do this include citric acid and baking soda. The third component is water, which serves as a filler and medium for the reaction.
The step-by-step algorithm for manufacturing a home converter is as follows:
add 1.3-1.5 liters of citric acid to 1 liter of water;
Citric acid can be replaced in the recipe with oxalic acid. The technology for preparing anti-rust with this organic acid does not change.
Purification of elements by complete immersion in phosphoric acid
If you have a sufficient amount of the product, you can use the full immersion method to remove rust. To do this, you first need to degrease the element to be cleaned using a detergent composition, and then wash it thoroughly. After this, pour 100 grams of an 85% acid solution into the prepared container and add 1 liter of water. The element to be cleaned is immersed in the solution for one hour, stirring the product periodically. Then the product must be removed and washed well. After such cleaning, the product is washed with another solution consisting of 50% water, 2% ammonia and 48% alcohol. Then the product is washed again with plain water and dried. All stages must be performed in strict sequence. If you do not degrease the product, the cleaning will be uneven, since the product may not corrode normal stains, and additional cleaning will be required. In this way, you can clean any products with varying degrees of rust, but the thicker the coating, the more time you need to keep the element in the solution. If the element is not dried after washing, hydroxide appears on it. Drying can be done using any method.
Rust converter for metal structures
Issues of protecting metal from corrosion worried people long before our time. About two thousand years ago, our ancestors used lead white and plaster to protect iron nail heads from rust. Since then, the production and use of the metal has increased manifold, and extensive knowledge and research has been accumulated on the nature of corrosion and methods of combating it. And if only a few decades ago only a highly specialized circle of people could know and use anti-corrosion treatment methods, now some of these methods are available to any consumer. To prepare metal surfaces for painting or repair previously painted parts with traces of corrosion, use a rust converter for metal structures.
About metal corrosion
The most famous process for the destruction of metallic iron is electrochemical corrosion. The interaction between iron and the surrounding electrolytically conductive environment results in the formation of hydrated iron oxide or rust. This happens due to the desire of the metal produced from ore to return to a more stable state with less energy.
Electrochemical corrosion in action
The generally accepted mechanism of this type of corrosion is explained by the laws of electrochemical kinetics and is characterized by the speed of electrode processes - anodic and cathodic. When metal surfaces come into contact with electrolyte solutions, iron ions pass into solution (anode process) with their subsequent release on the metal surface in the form of neutral particles (cathode process).
The most common destruction of metals is under the influence of the atmosphere, since 80% of metal structures are located in the open air. The influence of water adsorbed from the air (the influence of air humidity) and oxygen penetrating the metal-water interface create conditions for electrochemical corrosion. Additional factors that enhance the effect of destruction are aggressive gases and mechanical impurities. Thus, in industrial areas with high humidity, the corrosion rate is higher.
Rust converter: composition and application
Application of corrosion modifiers
A rust converter for steel structures is a special composition designed to transform the corrosive layer into an easy-to-clean loose surface or into a primer coating for subsequent painting with paints and varnishes. It is used in situations where it is impossible to use other methods (mechanical and physical-chemical) for removing rust. The recommended thickness of rust subject to modifiers should not exceed 100 microns, however, some gel solutions can handle greater thicknesses. Coating paint and varnish materials must have high adhesive ability to the converted layer and not enter into an electrochemical reaction with it.
Converters of corrosion products are conventionally divided into:
Components of rust converter formulations:
- Phosphoric acid is included in almost all rust removal and conversion products. Forms a protective layer of water-insoluble phosphate film, the composition of which promotes increased adhesion of paints and varnishes. If the integrity of the paint film and phosphate layer is damaged, the spread of corrosion is localized in the damaged area. Phosphoric acid has little effect on scale and non-hydrated oxides (magnetite, hematite and others). One-component compositions require rinsing before painting. Phosphoric acid is used as the main component in making your own rust converter.
- Tannin (hydroxycarboxylic acids). Converts iron oxide into tannate complexes, which firmly adhere rust particles at the molecular level to each other and to a clean layer of metal. In addition, tannin has inhibitory properties. Modifiers that use plant tannins as a basis are called neutral.
- Zinc monophosphate together with phosphoric acid form a zinc phosphate layer. Zinc has a protective function because it has a more positive electrode potential than iron, which is why iron enters into an electrochemical reaction with electrolytes more actively.
- Adsorption and passivating inhibitors are designed to slow down corrosion processes. The passivation process occurs with the help of substances with oxidizing properties. A protective film containing a passivating composition (the most popular use of chromates) shifts the corrosion potential in a positive direction, which slows down the destruction of the metal. Adsorption inhibitors are surfactants that form an additional film on the oxide layer, increasing resistance to the corrosion process.
- Aqueous compositions may contain dispersions of film-forming substances (alkyd, epoxy and others).
Best Rust Converters
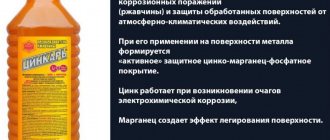
Rust converter Tsinkar
Rust converter Zinkar, instructions for use
Zincar is a multi-component rust modifier based on orthophosphoric acid with compounds of zinc and manganese salts.
- Pre-clean the surface of grease and then remove loose rust using a grinder, sandpaper and a brush.
- Treat the area with rust converter using a brush, roller or spray gun. Moisture should not be allowed to enter the surface during the treatment and operation of the solution. When working, use protective equipment - gloves, respirator, goggles.
- If, after drying, the transformed layer has a heterogeneous and loose appearance with red spots, then the first two steps should be repeated until a uniform, dense layer of gray color is obtained
- Before painting, wipe the surface with a rag, remove any remaining dust and rust with a brush, and dry with a hairdryer if necessary.
The success of using rust modifiers depends on the uniformity and homogeneity of the layer of corrosion products and their phase composition. Different prices and similar recipes for rust-to-primer converters with zinc - Khimik, Pantsir, Kolchuga.
Neutral rust converter IFKHAN 58R
The basis of this modifier consists of vegetable tannins with inhibitors and functional additives, without mineral acids. Converts a rusty layer into a black primer. Can be used as a stand-alone coating in the absence of direct exposure to precipitation. Used for preparing steel structures, pipes and fittings for concreting and painting.
How to properly remove rust using phosphoric acid
The advantage of phosphoric acid is that it removes loose rust and also forms a thin layer that serves as protection. The acid is capable of corroding rust and providing a protective film in the form of an oily surface.
Methods for removing rust depend on the degree of damage:
- cleansing of elements by complete immersion in the product;
- surface treatment using a roller or spray;
- applied to metal products after mechanical cleaning.